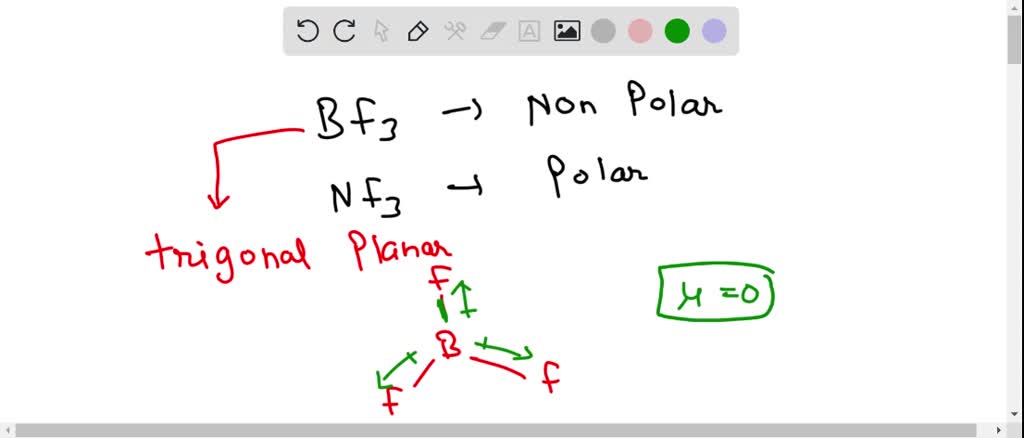
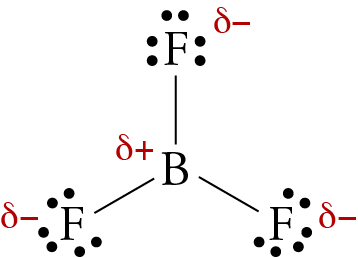
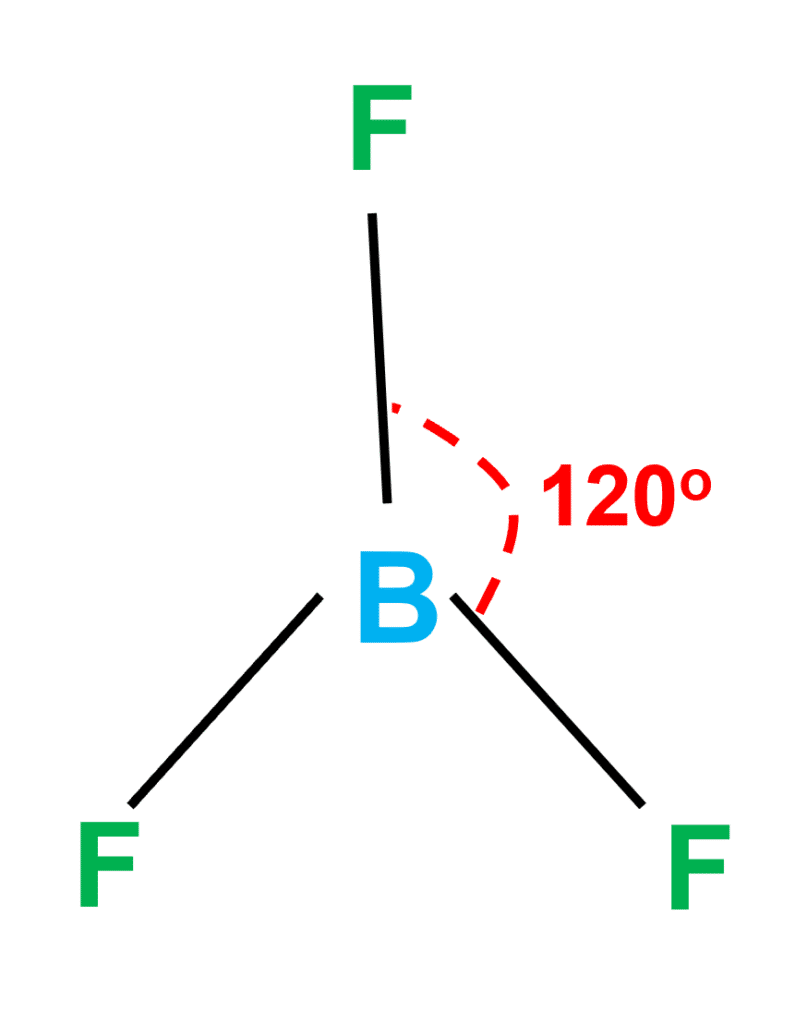
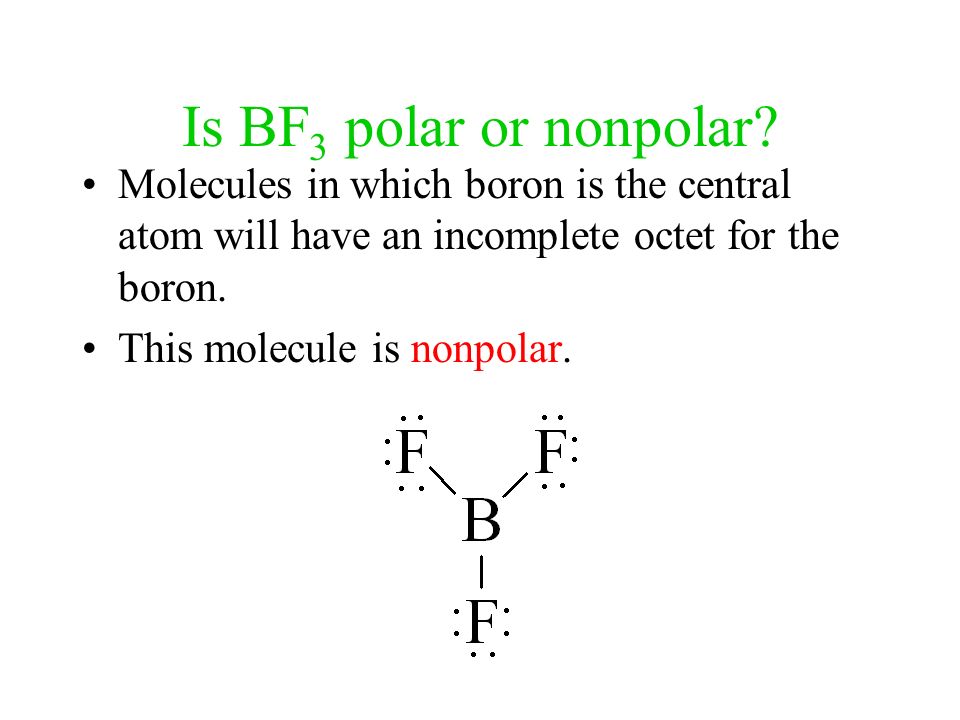
Ammonia (NH3) is a polar molecule while boron trifluoride (BF3), is a nonpolar molecule. What is the difference in the polarity of these compounds? - Quora
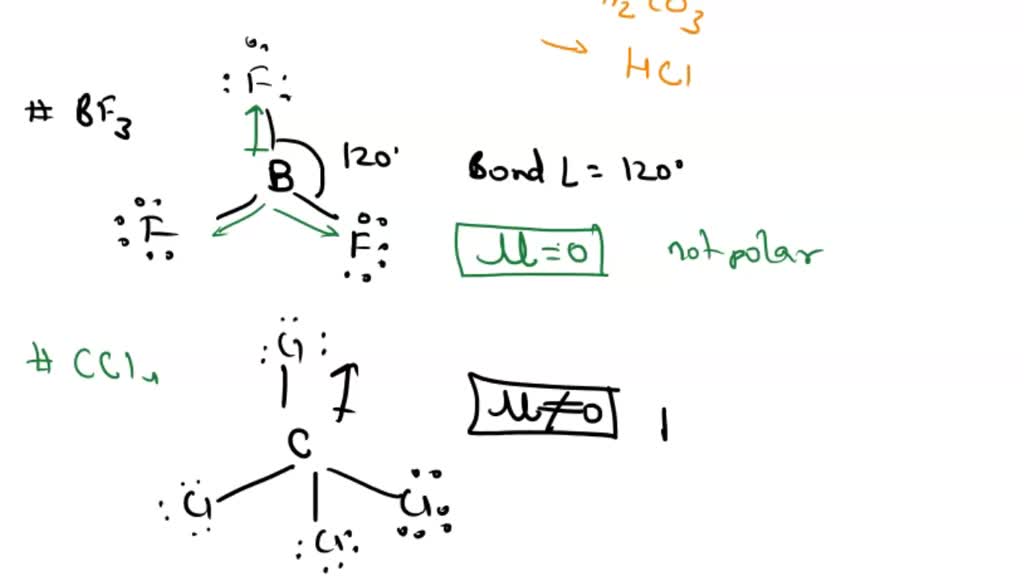
SOLVED: Determine the bond polarity, molecular polarity; electron geometry molecular geometry and bond angles and Lewis structure: 1 BF3 2 CCI 3 PCI; HCO 5 HCI

Boron Trifluoride BF3 is a non polar molecule whereas ammonia NH3is a polar molecule. The difference in polarity is related to the fact that