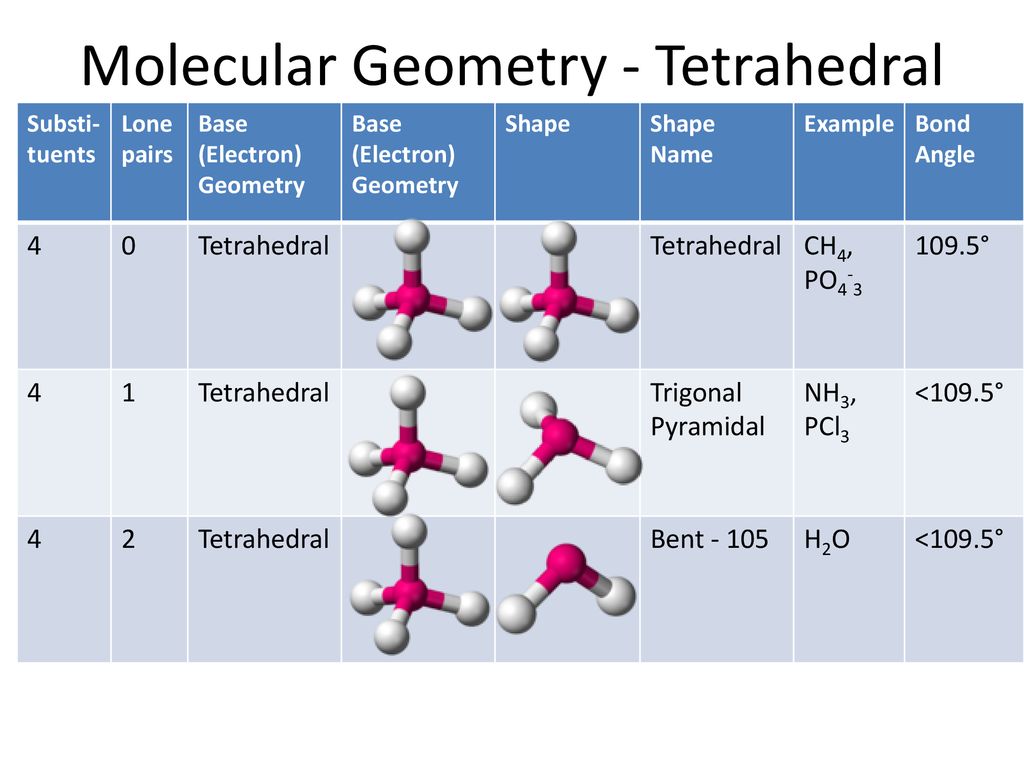
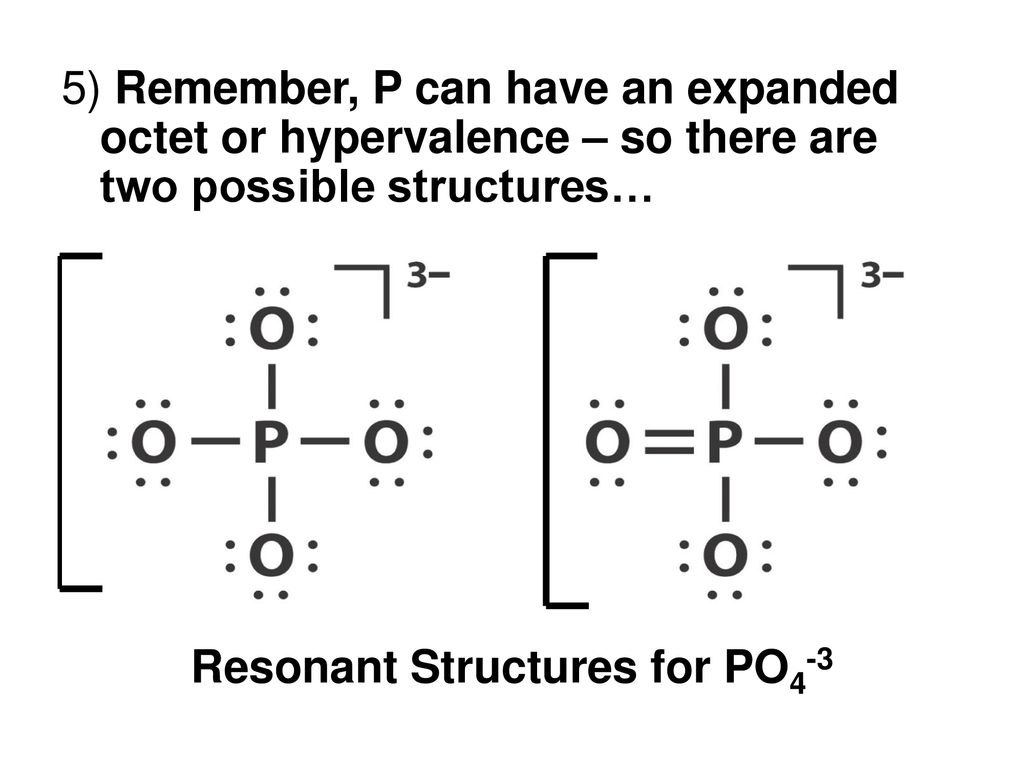
PO43- Lewis Structure (Phosphate Ion) | PO43- Lewis Structure (Phosphate Ion) Did you know that Phosphorus can have expanded orbitals and can accommodate more than 8 electrons in its outer... | By
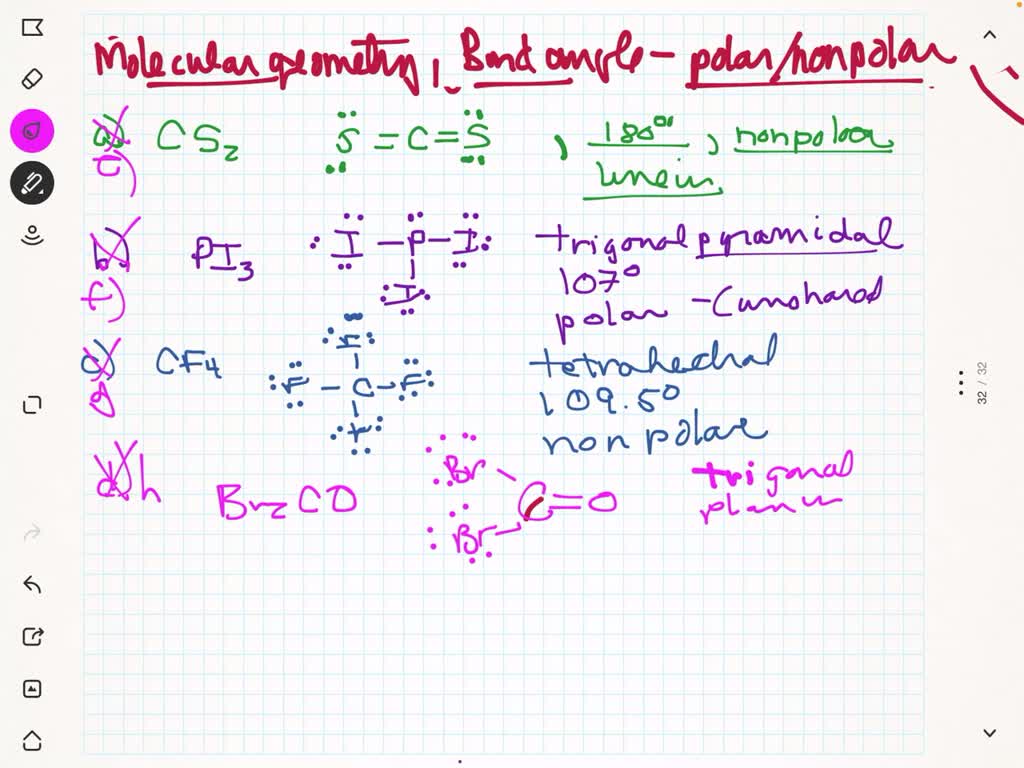
SOLVED: Molecular e) CS2 f) PI3 g) CF4 h) Br2CO (C is central) i) H2S j) PO43- k) SO32- 2) For compounds e) through i) above: a) Give the molecular geometry. b)
which of the following species is isostructural ,planar and non polar 1.ClF3,BrF3,xeo3 2. clo4^ ,so4^2 ,po4 ^3 3.I^ _3,Hgcl2, xef2 4.I^+_3,xef3^+ ,s_3^2

Draw the Lewis structure for PO43- and determine its electron and molecular geometries. | Homework.Study.com